It's 3:30 Monday morning, I've been in the lab for 42 hours now (with a break for dinner on Saturday and some football on Sunday night).
Things are going excellently!!! I'm currently collecting data on the 11th of my 12 samples! The precision on the first 10 range from great to world-record, so that's pretty good. :) This sample is looking like it's going to give us good data as well. I still need to measure some additional information to calculate the ages of the garnet, but these 12 are by far the most important and the most difficult to run...so it should be coasting from here.
One of these days I'll post a blog about what's going on in my life that isn't work related (not much, but there are a few things), but for now....as I promised in a previous post, here's a quick description of the TIMS: a sample of a pure (well, pure-ish) element is loaded onto a filament that is exactly like a light bulb filament. We heat up the filament by passing a current through it and turn the sample into ions. These ions are passed through a magnetic field created by this 937lb electromagnet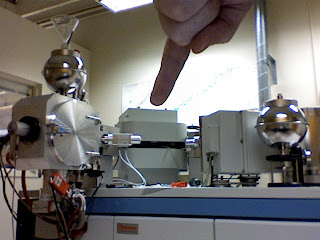

From the magnetic field the ion beams are bent and separated by their masses. Imagine two cars coming into a sharp turn. One, a Ferrari, will take the turn much tighter while the other, a Mack truck, will not be able to turn as quickly and will make a much wider turn. It's exactly the same principle with mass spectrometry. Light ions get bent to the inside of the flight tube and heavy ions to the outside. From there the isotopes are collected separately and the ratio can be calculated (discussion of this point will be saved for a later time).
This is where we load that barrel of samples we saw in the previous post.

Because we have to run at a very high vacuum, we have two vacuum pumps attached to the machine below where my finger is pointing in the last picture. To help these out and to get a much stronger and more consistent vacuum we use liquid nitrogen. The liquid nitrogen goes into the machine and literally freezes the water, carbon dioxide and other molecules out of the air in the machine, leading to lower vapor pressure. This silver ball is full of liquid nitrogen.

This is the computer I use to run the instrument. Dude, it's a Dell!

These lines I'm pointing at represent the amounts of each isotope I'm collecting at that time. In this case the highest blue one (trust me, it's blue) represents the ion neodymium-150 + oxygen-16.
And this is what I look like after 42 hours in the lab (note the smile....things are going that well):
 Sample 11 is running itself nicely. I'm off to bed (well, a sleeping bag on the floor near the TIMS). One more sample when I wake up and then it'll just be a short hop, skip and a jump until I get my masters (somewhat exaggerated).
Sample 11 is running itself nicely. I'm off to bed (well, a sleeping bag on the floor near the TIMS). One more sample when I wake up and then it'll just be a short hop, skip and a jump until I get my masters (somewhat exaggerated).

No comments:
Post a Comment